X-ray inspection machine for pharmaceuticals【for supplements】
Improving the quality and safety of supplements through high-precision inspection.
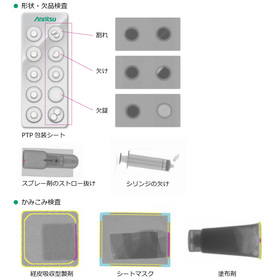
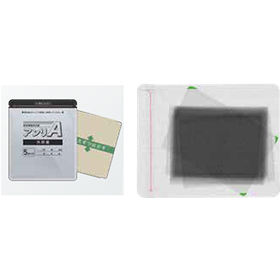
In the supplement industry, with the increasing health consciousness of consumers, the quality and safety of products are becoming increasingly important. Contamination of tablets with foreign substances or defects in shape can not only harm consumer health but also lead to a loss of trust in companies. X-ray inspection machines for pharmaceuticals can prevent these issues through high-precision inspections and ensure product quality. 【Usage Scenarios】 - Foreign substance inspection of tablets - Shape inspection of tablets - Inspection for missing items - Inspection for chipping 【Benefits of Implementation】 - Reduction of foreign substance contamination risks - Improvement of product quality - Enhancement of corporate reliability
basic information
【Features】 - High precision and high stability. Capable of performing multiple quality inspections simultaneously with one unit. - Suitable for inspecting opaque packaging materials such as double-sided aluminum PTP and transdermal patches. - Verified the impact of X-ray irradiation on formulation quality. - Designed to prevent X-ray leakage with a primary focus on operator safety. - Compliant with FDA 21CFR Part 11 (optional). - 24-hour support (Japan only). 【Our Strengths】 Our company is based on "measurement" technology and focuses on business development in the fields of information communication and food and pharmaceuticals. With over 20 years of technical expertise and sales performance, we provide optimal inspection solutions for our customers' products.
Price information
Please contact us.
Delivery Time
Model number/Brand name
KXE7510DGEKE
Applications/Examples of results
【Purpose】 ■ Comprehensive quality inspection of products (missing items, foreign substances, shape, entrapment, quantity, bubbles, etc.) ■ Internal quality inspection of opaque packaging materials 【Achievements】 Cumulative sales: Over 15,000 units* (entire comprehensive inspection solution) ■ Countries where products are shipped: Over 80 countries* (PQA business) *As of the end of March 2023 You can check in advance what inspections are possible with the X-ray inspection machine through sample testing. For more details, please refer to the PDF document or feel free to contact us.
Related Videos
Line up(1)
| Model number | overview |
|---|---|
| KXE7510DGEKE | Paper feeding machine |
catalog(16)
Download All Catalogs
Recommended products
Distributors
[Trusted Worldwide: Anritsu Inspection Systems] Anritsu inspection systems are used globally to ensure product safety and quality across a wide range of industries and applications. Key Features of Our Weighing & Inspection Solutions ●Contaminant Detection Detects foreign materials such as metal, bone, glass, ceramic, and more. ●Content Verification Checks for missing or excess items, presence/absence, and item counts. ●Package Inspection Identifies issues like product caught in seals and defective printing. ●Weight Inspection Performs quantitative checks and grading for consistent product quality. ●Production & Quality Management Enables centralized control, real-time monitoring, statistical analysis, and traceability through our proprietary system, QUICCA. [Supporting the Pharmaceutical Industry Since 1964] Anritsu delivered its first checkweigher to a pharmaceutical manufacturer in 1964. Inspired by the industry's strict standards for quality control, we have continuously evolved our technologies to meet advanced quality assurance needs. Today, Anritsu Infivis works closely with pharmaceutical partners to address complex challenges in manufacturing and compliance. [In-House Development for Superior Performance] All Anritsu products—including weighcells—are developed in-house. This commitment to innovation is reflected in the high performance of our electromagnetic force balance weighcells. [Enhancing Quality and Productivity in Pharma] Anritsu’s inspection technologies contribute to improved quality and efficiency in pharmaceutical manufacturing and R&D environments.